Your Questions Answered: Kidney Lab Results
Creatinine? Microalbuminuria? BUN? eGFR?
ASF will host a 60-minute, moderated Q&A discussion with Dr. Anjay Rastogi, M.D., Ph.D. to help Alport patients and families better understand the results of their renal labs (blood and urine).
Tuesday, October 5, 2021
9:00am Pacific
10:00am Mountain
11:00am Central
12:00pm Eastern
Free, pre-registration is required to join the virtual event and ask questions.
Dr. Rastogi, Professor and Clinical Chief Director of UCLA’s CORE Kidney Program, and member of ASF’s Medical Advisory Committee, will discuss what lab values mean, their implications for current and future health, and steps you can take when specific lab values are out of range.
A closed-captioned recording of this Webinar is now available on our YouTube channel.

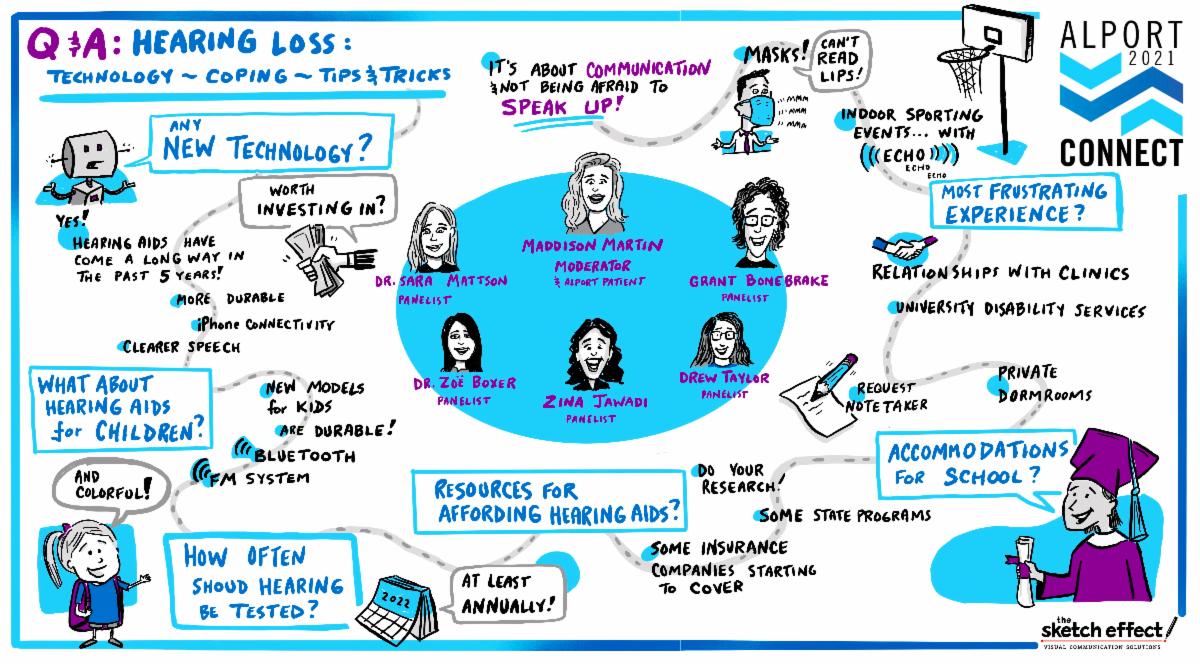
Now Available: Hearing Loss Panel Recording
Feelings of isolation, fatigue, and frustration are common experiences for people living with Alport-associated hearing loss. The 2019 Alport syndrome Voice of the Patient Report clearly documented the impact hearing loss has on many members of our community. At last month’s Alport Connect 2021 meeting, ASF brought together patients and audiological experts to discuss the latest technology, coping mechanisms, and tips and tricks to make living with hearing loss more manageable.
If you missed this Q&A panel session, or want to watch it again, click the button below to view the closed-captioned recording. It features 2 patient panelists, 2 audiologists, and a Board Member from the Hearing Loss Association of America.
Accomplished book illustrator and sketch artist, John Whitworth, joined us to illustrate this panel in real-time. Click on the illustration below to view the a full-size image of key takeaways.
New Alport Syndrome Research
A September 2021 Scientific Reports article documenting new Alport research in China expands upon a potential reason why some patients/families that have a clear traditional diagnosis of Alport syndrome still exhibit negative genetic test results for Alport syndrome.
ASF’s volunteer Research Program Chair and Board Member, André Weinstock, PhD, MSAS provides a layman’s summary at this link.
An article recently published in Kidney International Reports discusses use of induced pluripotent stem cells as a model for Alport syndrome. Induced pluripotent stem cells start as simple skin cells, are reprogrammed by researchers to become stem cells, and are then directed to become podocytes all in a dish. This new model was found to accurately model Alport syndrome and provides a method of endlessly generating podocytes for use in research.
Joseph Lagas, Doctoral Candidate in Molecular Biology (Washington University in St. Louis) and member of ASF’s Emerging Leadership Counsel generously agreed to provide a layman’s summary at this link.

2021 Paul Silver Enrichment Award: Why Apply?
Click the “CC” button to activate closed-captioning on both videos.
ASF asked two 2020 recipients of the Paul Silver Enrichment Award (PSEA) to share why they applied and how the award funding helped them achieve their respective goals. Hear from Lindsay and Niklas by clicking their videos above.
The PSEA is open to individuals ages 16 to 22 years old living in the United States. A minimum of $1,000 will be awarded to the winning applicant/s. Application deadline: Friday, November 12, 2021
Alport Syndrome Clinical Trials Overview
ASF has recently received an influx of patient questions regarding clinical trials open to those with Alport syndrome.
Here’s a brief overview for all members of the Alport community:
Currently Enrolling Clinical Trials
- The AFFINITY Study (Chinook Therapeutics)
- The HERA Study (Sanofi-Genzyme)
- The FREEDOM-1 Study (Talaris Therapeutics)*
ASF’s Clinical Trials YouTube playlist includes recordings of educational webinars we hosted with all 3 aforementioned study sponsors to help Alport patients best understand each clinical trial.
*Please note FREEDOM-1 is open to patients pursuing kidney donation from a live donor, while AFFINITY and HERA are studying Alport-centric therapeutics.
Closed Recruitment
- The CARDINAL Study (Reata Pharmaceuticals).
Reata Pharmaceuticals’ New Drug Application (NDA) for bardoxolone methyl for the treatment of patients with chronic kidney disease caused by Alport syndrome is currently under review by the FDA. This is the first time any NDA of any sort has been filed specifically for Alport syndrome. The FDA action date for the application is scheduled for February 25, 2022.
A full review of up-to-date clinical trial information, including individual study criteria and study sites, can be found on our website.
Transplant Recipients: Invitation to Connect
Based on positive feedback about our “Direct Connect” virtual rooms as part of Alport Connect 2021 weekend, ASF will host a small virtual gathering of kidney transplant recipients on:
Tuesday, October 5th
4:00pm Pacific Time
5:00pm Mountain Time
6:00pm Central Time
7:00pm Eastern Time
This 60-minute facilitated discussion provides opportunity for Alport renal transplant recipients to share their experiences with follow-up care, immunosuppressant medications, COVID and vaccinations, and more.
To facilitate optimal conversation, space is limited.
*This event has passed and ASF will plan future Transplant meetings in Fall 2021*